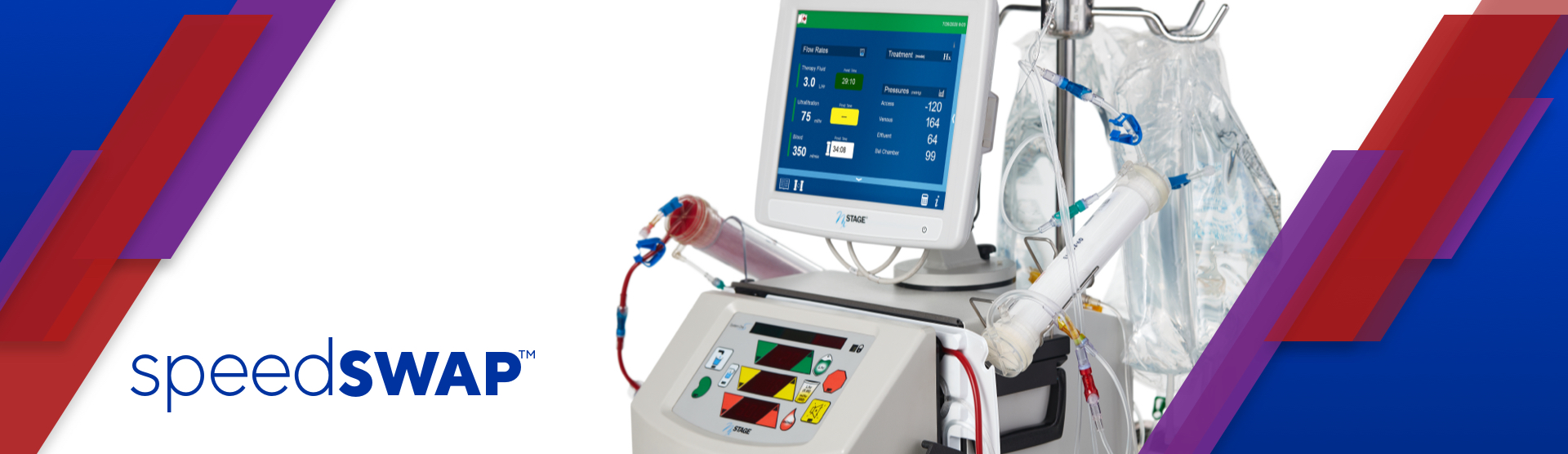
Risk Information
Kidney replacement therapy, as with any medical therapy, is not without risks. The decision of which therapy to use should be made by the physician, based on previous experience and on the individual facts and circumstances of the patient. There is no literature demonstrating one therapy is clinically better than another.9 NxViewTM is contraindicated as the sole method of monitoring a patient during treatment. The Speedswap cartridge can only be used with the following software versions and user guide:
- NxView Software 3.0 or higher
- SystemOneTM Cycler Software 4.14 or higher
- NxStage SystemOne User Guide NC4921 Rev. J r higher
References
- Joannidis M, Oudemans-van Straaten HM. Clinical Review: Patency of the Circuit in Continuous Renal Replacement Therapy. Crit Care 2007;11(4):218.
- NxStage System One Critical Care User Guide. NC4921 Rev. J. 2021.
- Data on file, 08/2021.
- Instructions for Use CAR-535/SSK-535. NC45-0926. Rev. E. 04/2021.
- Polaschegg HD. Mechanical Aspects of Dialysis; The Extracorporeal Circuit. Semin Dial 1005;8(5):299-304.
- Needlestick Prevention and Safety Act, 106th Congress, Public Law 106-430, 2000.
- Filter Membrane Webinars, part 1, www.nxstage.com. Data on file, February 2010, 3M GmbH. www.membrana.com
- Filter Membrane Webinars, part 2, www.nxstage.com. Data on file, May 2010, 3M GmbH. www.membrana.com
- D. M. Nash, S Przech, R. Wald, and D. O'Reilly, "Systematic review and meta-analysis of renal replacement therapy modalities for acute kidney injury in the intensive care init," Journal of Critical Care, vol. 41, pp. 138-144, 2017.
Caution
Federal law restricts this device to sale by or on the order of a physician.
© 2022 Fresenius Medical Care. All rights Reserved. Fresenius Medical Care, the triangle logo, NxStage, Speedswap, NxView, and System One are trademarks of Fresenius Medical Care Holdings, Inc. or its affiliated companies. All other trademarks are the property of their respective owners. P/N 104988-01 Rev A 04/2022 APM4464 Rev A